2hi G H2 G I2 G
2HIg H2g I2g Kp for the reaction is 0016. Calculate the concentrations of the gases at.

Solved 8 Select The Correct Equilibrium Constant Chegg Com
The concentrations of each specie is.

. Calculate the concentrations of the gases at. Express the equilibrium constant to three significant digits. Calculate the equilibrium constant of the reaction.
H₂ g I₂g 2HIg From that equilibrium equation you can fnd the expression of the equlibrium constant Kc as. 2HI g-- H2 g I2 g The rate law for the decomposition of HI is rk HI2. There are 2 Hydrogen atoms and 2 Iodine atoms on the right so the have to be 2 of each on the left as well so there are 2 H I molecules on the left.
If 0200 atm of HIg is sealed in a flask what is the pressure of each gas when equilibrium is established. 2HI gH2 gI2 g2HI gH2 gI2 g Use the simulation to find the initial concentration HI0 HI0 and the rate constant kk for the reaction. Please click on the image of the file to open it.
The calculations and explanations to answer the three questions are in the pdf file attached. Hydrogen iodide can decompose into hydrogen and iodine gases. Museum J1 Mastitis Research Barn J1 Mateer C4 Materials.
碘与氢气反应的热化学方程式是①I2g H2g 2HIg ΔH 9 48 kJmol1 ②I2s H2g 2HIg ΔH. The equilibrium concentration of H2 and I2 is 0165 M. H2 g I2 g 2HI g ΔHº 518 KJ un aumento de temperatura aumentaría la cantidad en equilibrio de HI g.
The rate of the rxn was found experimentally to b 25 x 104 molL x s where the HI concentration was 00558 M. H2g I2g 2HIg. 2HI g H2 g I2 gThe rate equation bartleby 2HI g H2 g I2 gThe rate equation for the reaction is rate k HI²At 629 K and at a concentration of 200 mol dm-3 the rate of decomposition of hydrogen iodide 240 x 10-5mol dm-3s-2.
H2 152 075 M. The equilibrium constant Kc for the reaction H2g I2g 2HIg is 543 at 430 C. The reaction H2 g I2 g 2HI g may occur by the mechanism shown above.
In the reaction H2 g I2 g 2HI g the amounts of H2 I2 and HI are 02g92525g and 448g respectively at equilibrium at a certain temperature. The term Kc refers to the equilibrium constant for a aqueous phase reaction. 2H I g H 2g I 2g Explanation.
2HI g H2 g I2 g Keq 0016 Initially a container contains 060 M HI 0038 M H2 and 015 M I2 at equilibrium. August 11 2019 ˇ ˆ. The equilibrium concentration of HI is 117 M.
You may want to reference Pages 638 - 640 Section 155 while completing this problem. En la siguiente reacción. I2 152 075 M.
Butler Ag Engineering H2 Butler Farm F1 Calder Square II D6 Carnegie C5 Carpenter C4 CEDAR C4 Center for Sustainability I4 Centralized Biological Lab H6 Central Milk Testing Lab I1. Class 11 Chemistry Equilibrium Law of Chemical Equilibrium and Equilibrium Constant In the reaction H2 g I2 g 2HI g the. 对于可逆反应H2gI2g2HIg在温度一定下由H2g与I2g开始反应下列说法正确的是双选 A.
2HI gH2 gI2 g At equilibrium it is found that HI 358103 M H2 486104 M and I2 486104 M. 3 para a pergunta Urgentee. See the answer Consider the second-order reaction.
What is the rate law predicted by the mechanism. Are these values ready to plug in to the equation or because Chemistry At a particular temperature K375 for the following reaction. What is the value of keq for the equilibrium below.
At the start of the reaction the was 714 mole of H2 0984 mole of I2 and 0886 mole of HI in a 280 L reaction chamber. When equilibrium constant is reached the concentration of H2 g is chem A flexible container at an initial volume of 411 L contains 751 mol of gas. Hydrogen iodide decomposes according to the reaction 2HI gH2 g I2 g A sealed 150 L container initially holds 00623 moles of H2 00414 moles of I2 and 0244 moles of HI at 703K.
At the start of the reaction the was 714 mole of H2 0984 mole of I2 and 0886 mole of HI in a 280 L reaction chamber. The value of keq for the equilibrium h2 g i2 g 2hi g is 794 at 25 c. Livestock Testing Lab I2 Lubert N1 Maintenance I Pollock F5 MascaroSteiniger Turfgrass Equip.
Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy. Setting up the ICE table. The activation energy for the reaction 2HIg H2 I2g is 2095 kJ mol1 at 581K.
The equilibrium constant Kc for the reaction H2g I2g 2HIg is 543 at 430 C. ACalculate the rate constant k for the reaction at 629K. This problem has been solved.
Express your answer in moles per liters This problem has been solved. What is the value of Kc at this temperature. See the answer See the answer done loading.
Y la expresión de la constante de equilibrio para este sistema es. 12 h2 g 12 i2 g hi g 2. 碘与氢气反应的热化学方程式是 ①I2g H2g 2HIg Δ.
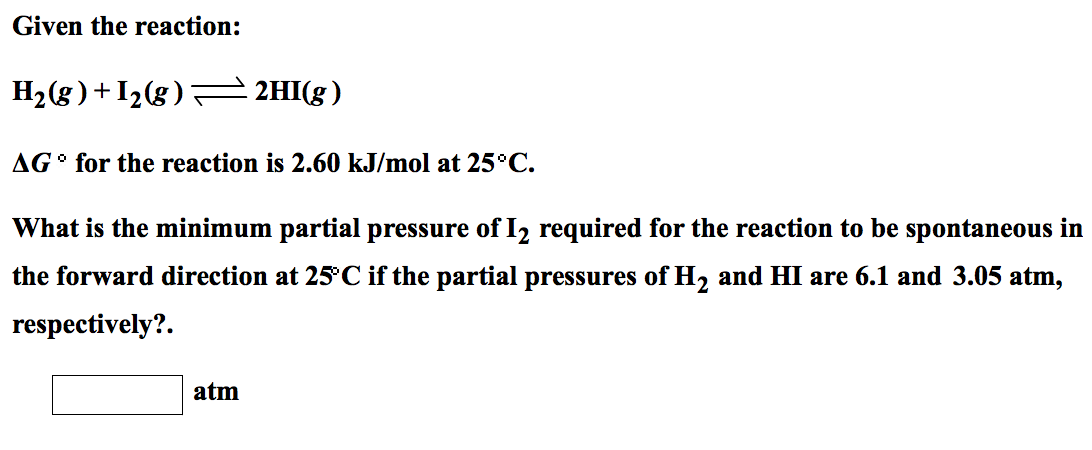
Solved Given The Reaction H2 G I2 G 2hi G Delta G Chegg Com
![]()
Diketahui Reaksi H2 G I2 G 2hi G Jika Diet
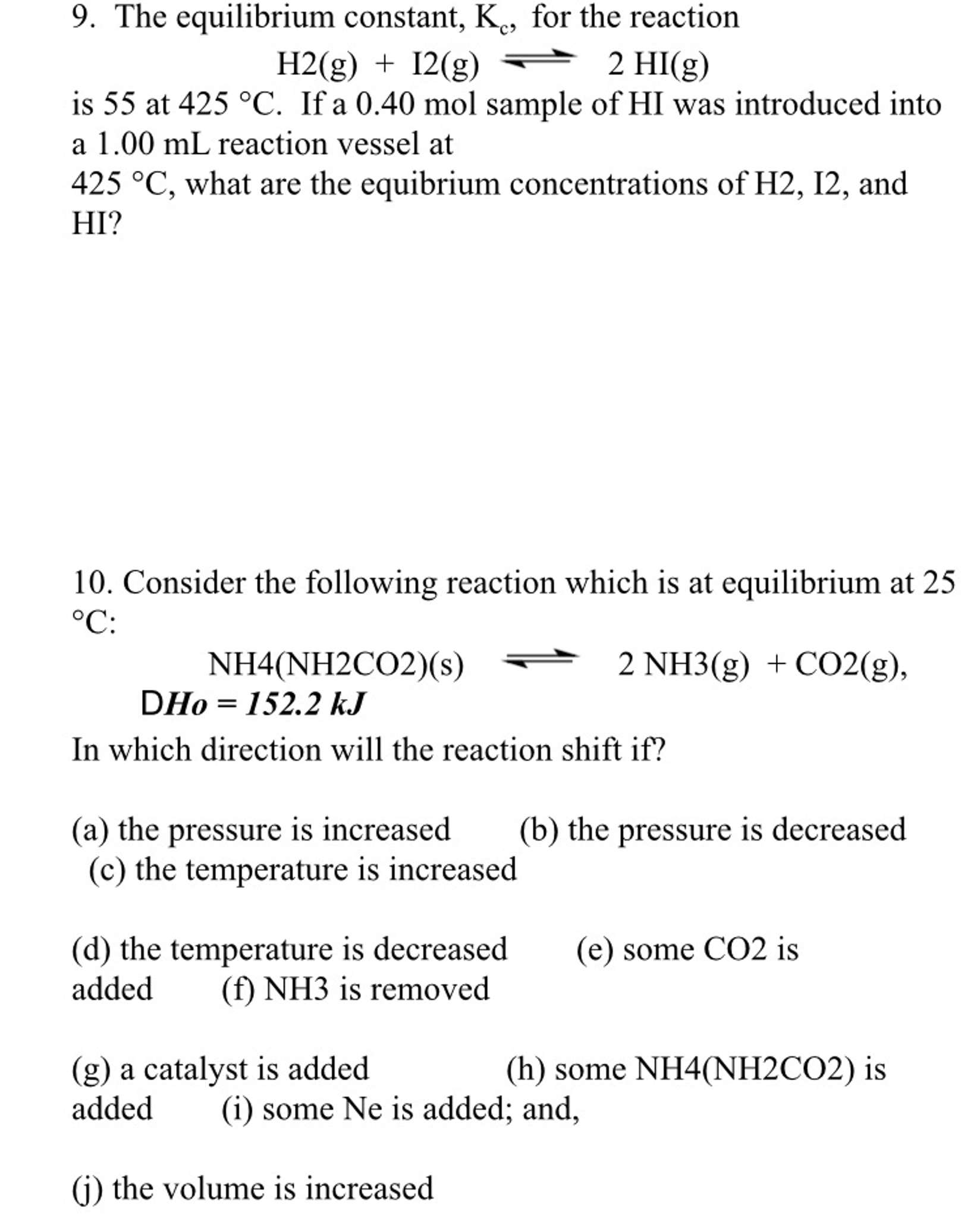
Solved The Equilibrium Constant K C For The Reaction H2 G Chegg Com
For The Reaction H2 G I2 G 2hi G The Rate Of Reaction Is Expressed As Sarthaks Econnect Largest Online Education Community

Solved 3 The Following Reaction Mechanism For H2 G Chegg Com

Pada Reaksi Kesetimbangan H2 G I2 G Lt Gt 2 Hi G Kearah Mana Kesetimbangan Bergeser Brainly Co Id
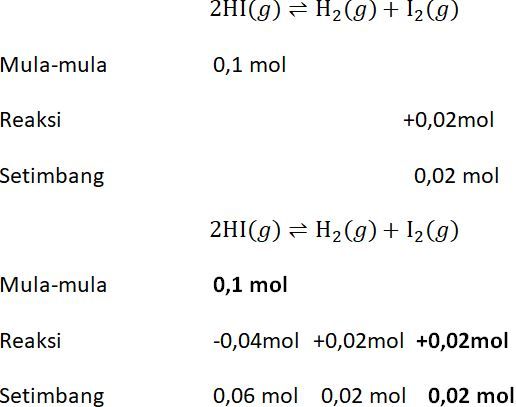
Sebanyak 0 1 Mol Hi Dimasukkan Ke Dalam Labu 1 Lit

Chemical Equilibrium Part Ppt Download

Pada Reaksi Kesetimbangan I2 G H2 G 2 Hi G Berlangsung Dalam Wadah 2 Liter Banyaknya Mol Brainly Co Id

Solved 6 Select The Correct Rate Expression For 2hi G Chegg Com
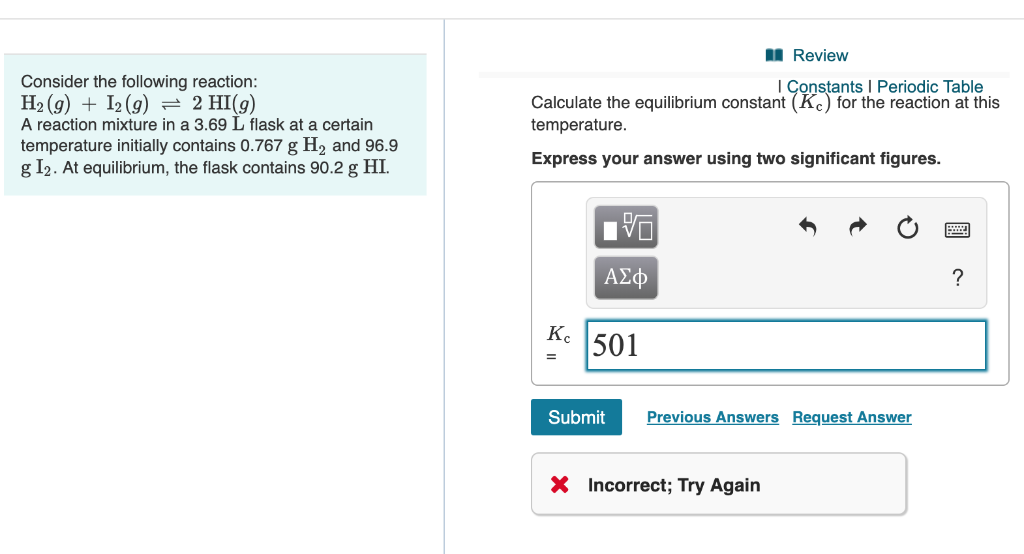
Solved Consider The Following Reaction H2 G I2 G 2hi G Chegg Com
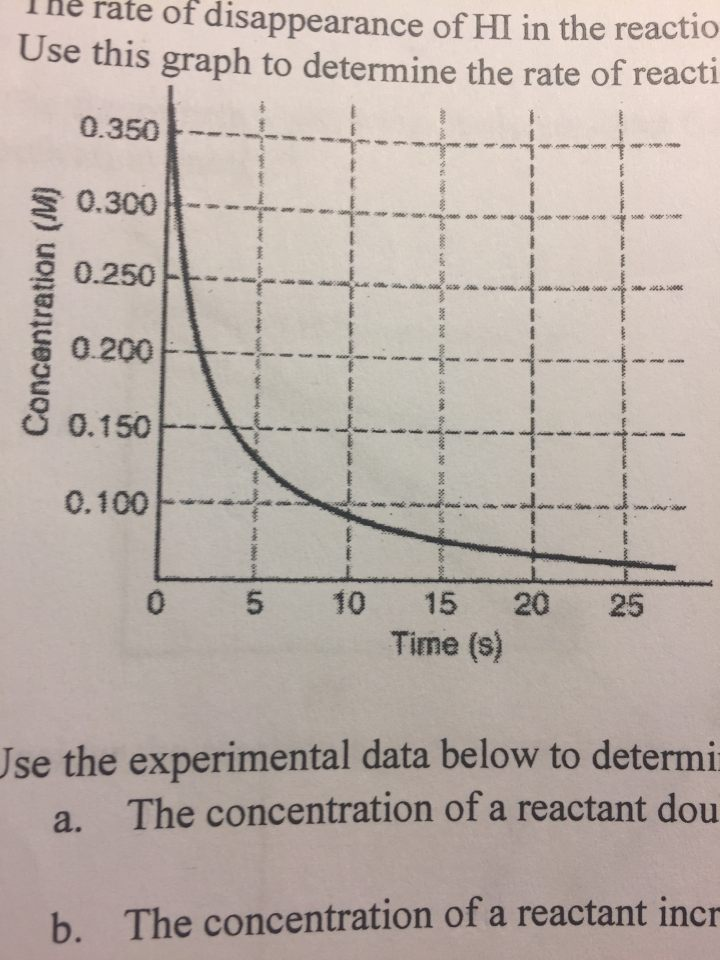
Solved The Rate Of Dissapearance Of Hi In The Reaction Chegg Com

Diketahui Reaksi H2 G I2 G 2hi G Kc 50 Jika Konsentrasi Hi 0 5 M Dan H2 Brainly Co Id
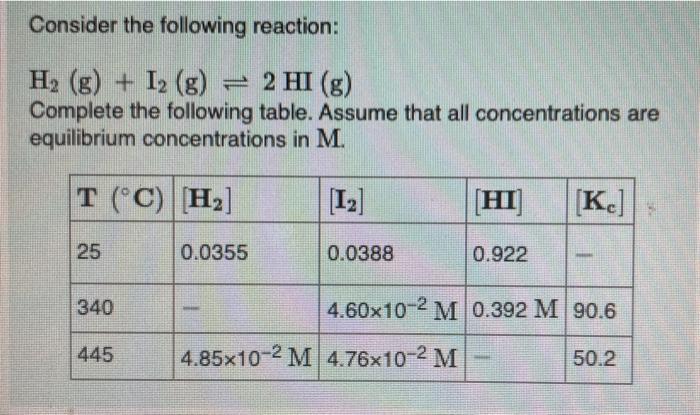
Solved Consider The Following Reaction H2 G I2 G 2 Chegg Com
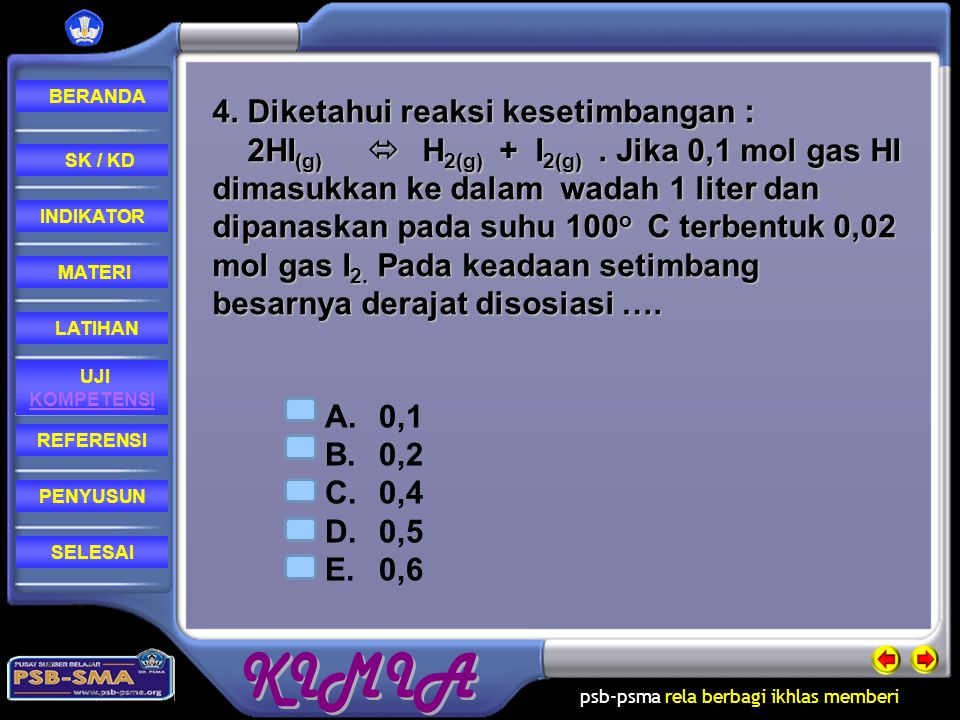
Hubungan Kuantitatif Antara Pereaksi Dengan Hasil Reaksi Dari Suatu Reaksi Kesetimbangan Ppt Download

Solved A Student Ran The Following Reaction In The Chegg Com

Solved A Student Ran The Following Reaction In The Chegg Com
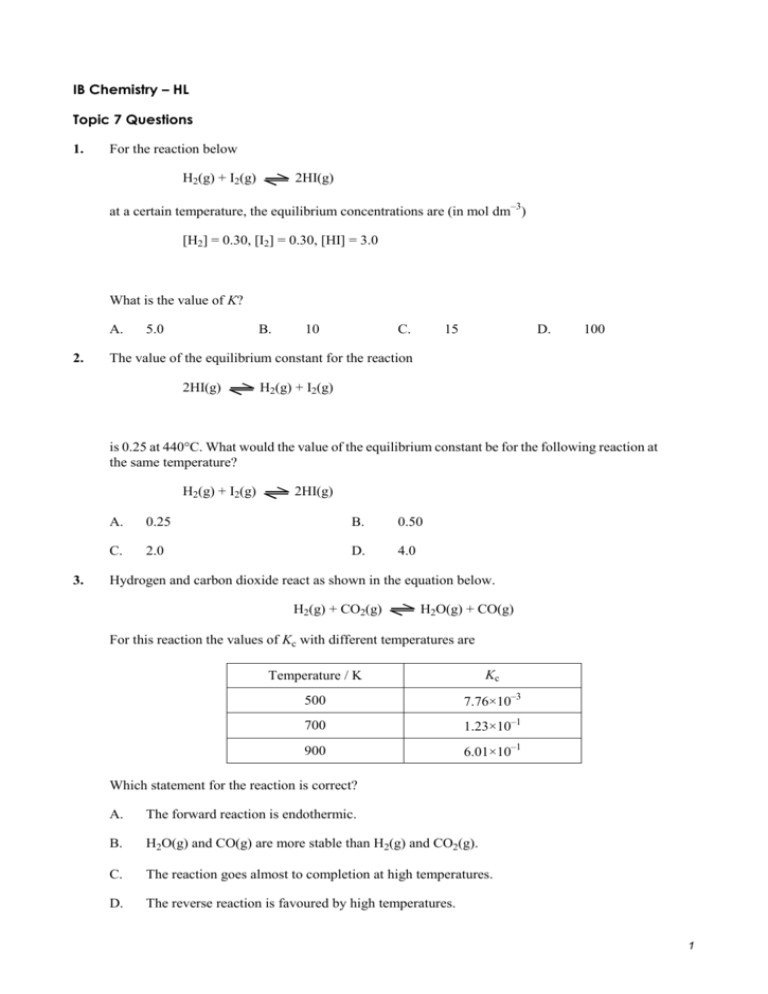
Comments
Post a Comment